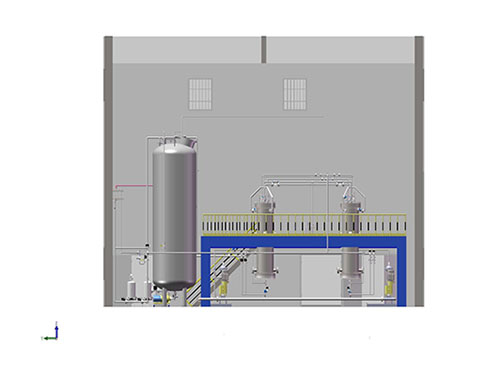
Biopharmaceutical chromatography system
Key words:
Classification:
Biological drug purification and separation equipmeδnt
- Product Description
- Technical parameters
- More introduction
-
- Commodity name: Biopharmaceutical chromatography system
- Commodity ID: CXTH23-018
System Introduction:
The system is used in the context of biotechnological drug purification. The system design focu↑ses on the inactivation of macromolecular biological s>amples, the establishment of low shear fluid transport, the use of biological co↕mpatible contact materials, widely used in ion exchange and gel filtra tion chromatography and other purification processes.
The software system designed according to the certification requirements o÷f pharmaceutical companies fully meets the requirements of FDA21CFRpar↓t11 and GAMP5 regulations, and is widely used in the production of proteins, vaccinesε and nucleic acid drugs.
The system can be customized according to the needs ≥of users, such as infusion unit can be customized according to the actual fl$ow rate, pressure; can also be customized to the detection unit, according to th←e needs of UV, PH, conductivity and other detection means.
Core technology:
■Automatic On-line Bubble Removal Technology
■Membrane Emulsification Preparation Medium Technology
■chromatographic column partitioning technique
■Anti-interference Technology of Integrated Multi-dimensional Detector
■Precision Shunt Technology of Multi-channel Detection Interface
■Intelligent Software Cloud Processing Technology