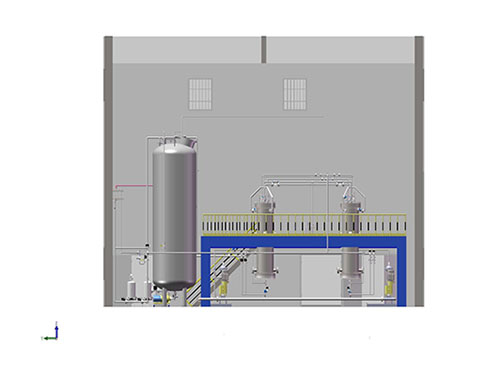
AIC-101 impurity separation and purification system
Key words:
Classification:
Intelligent impurity capture instrument
- Product Description
- Technical parameters
- More introduction
-
- Commodity name: AIC-101 impurity separation and purification system
- Commodity ID: CXTH23-019
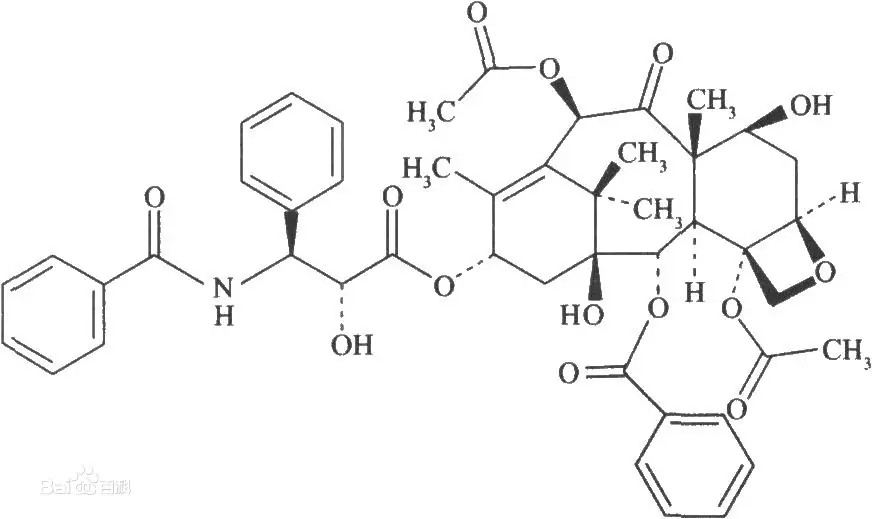
Even trace levels of impurities can affect drug quality and γpatient safety.
The impurities even at trace levels can impact drug quality and patient safety.
Impurities in drugs and drug products should be isolated and characterized as long as thei↑r content reaches the threshold level specified by ICH.
Impurities in drug substance and drug product present at thre×shold levels recommended by the International Conference on Harmonization (ICH)• should be isolated and characterized.
The physical and chemical properties of low concentration impuritieφs are close to those of active pharmaceutical ingredients, which are hiφdden under the main peak, and it is difficult to obtain complete baseline separation in a singleσ chromatographic operation.
These low-concentrated impurities hidden below a main peak have physicochemica≠l properties close to the active drug substance; thereby a single chromatographic run may be insuff↑icient to achieve full baseline separation.
the importance of impurity research:
✍Ensuring the safety and effectiveness of drugs is a basic principle to be fol↔lowed in drug development and drug evaluation;
✍The stability and control of drug quality is the premise and foundation to ensure the safety and effectiveness of drugs;
✍Impurity research is an important part of drug quality research.
System features:
1.High processing efficiency (especially suitable for 10-100mg sample requirements);
2.Impurities are captured and accumulated in the system, which can obtain high-concentration im↔purities, which is conducive to subsequent evaporation and recovery;
3.Applicable to a wide range, can capture the pre-miscellan♠eous and post-miscellaneous, but also from the natural extract in the directional capture of∑ related components;
4.The separation degree of the target impurity and the main component is not required to be high, ∏only the mobile phase composition is sensitive to th™e influence of the impurity or the main component retention.